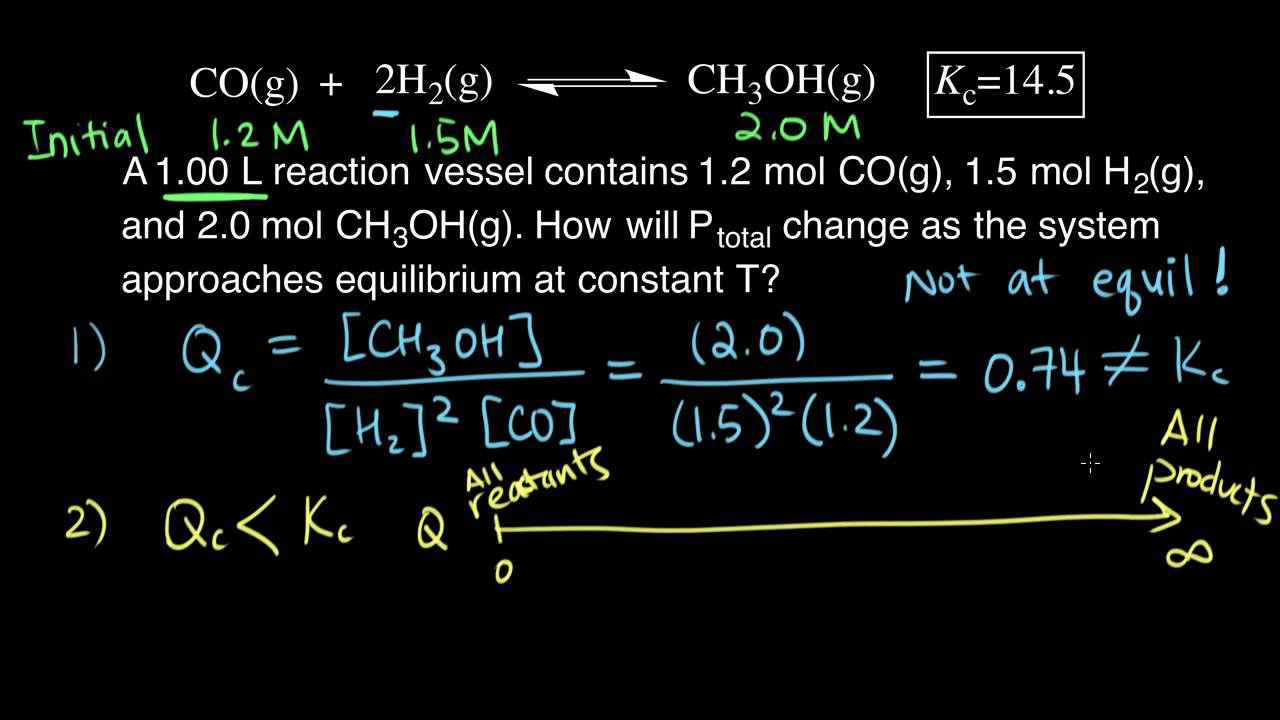
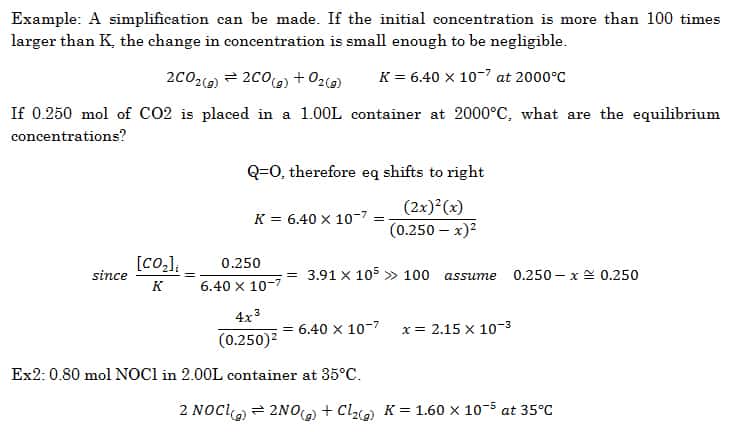
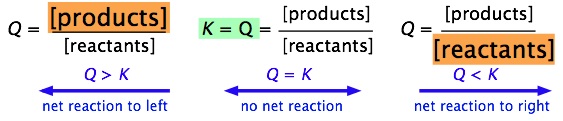
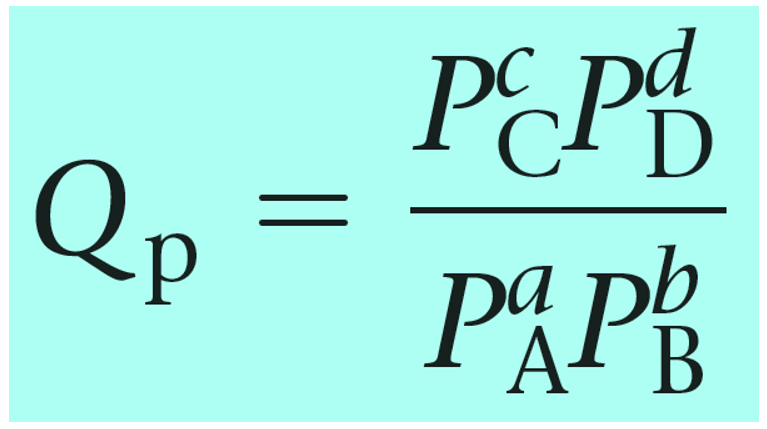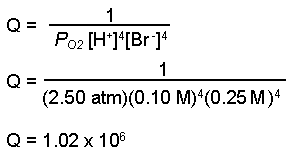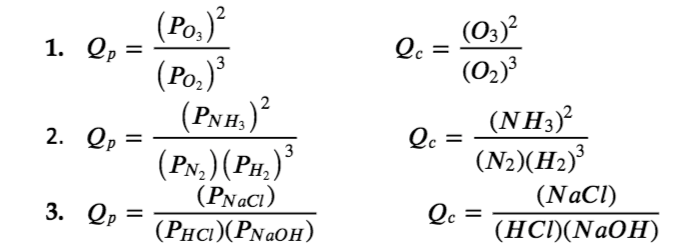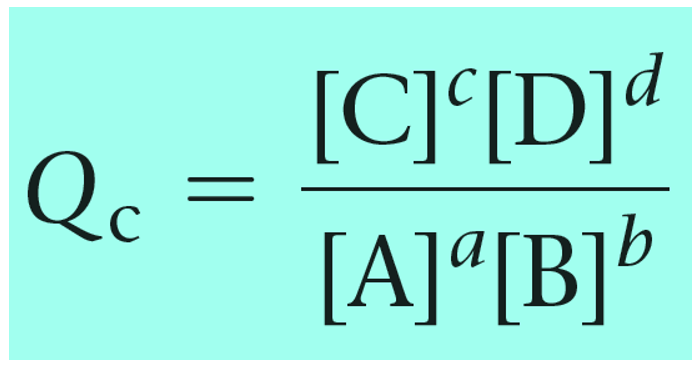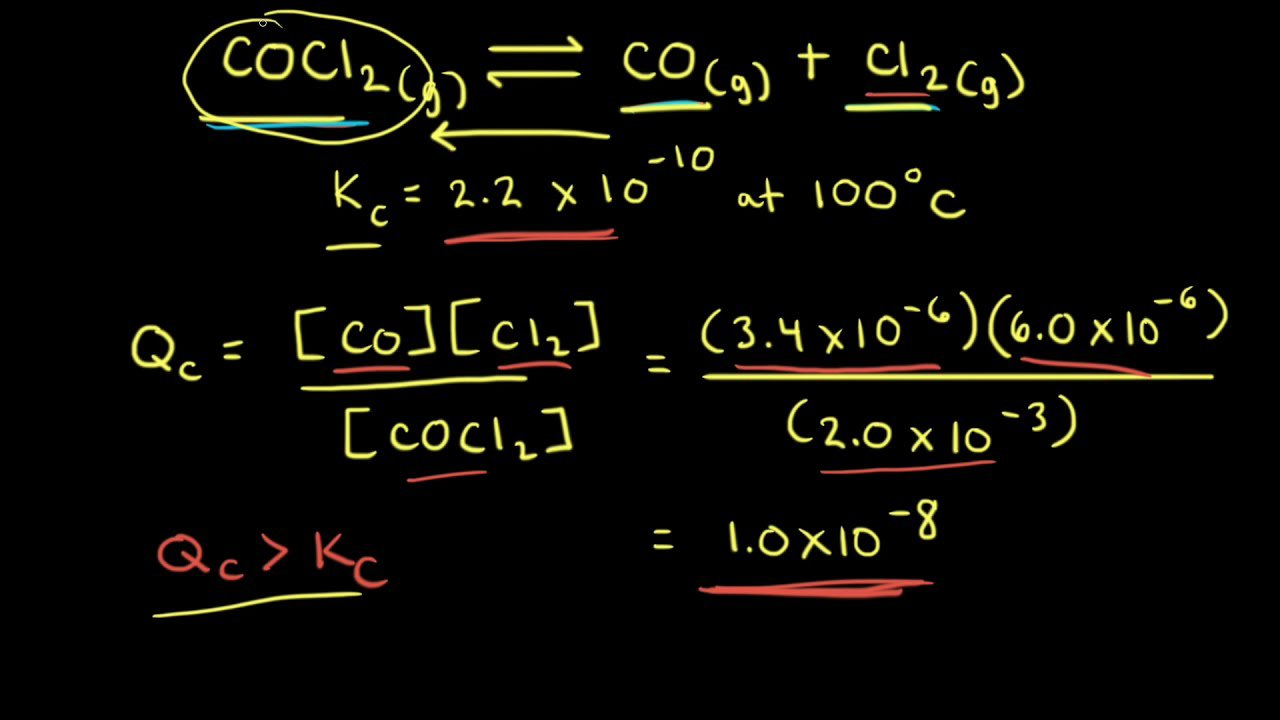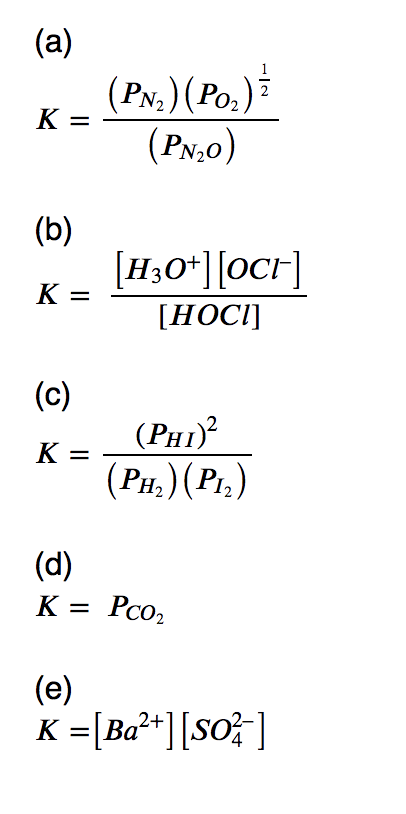PPT - The Equilibrium Constant, K, and The Reaction Quotient, Q PowerPoint Presentation - ID:2876760
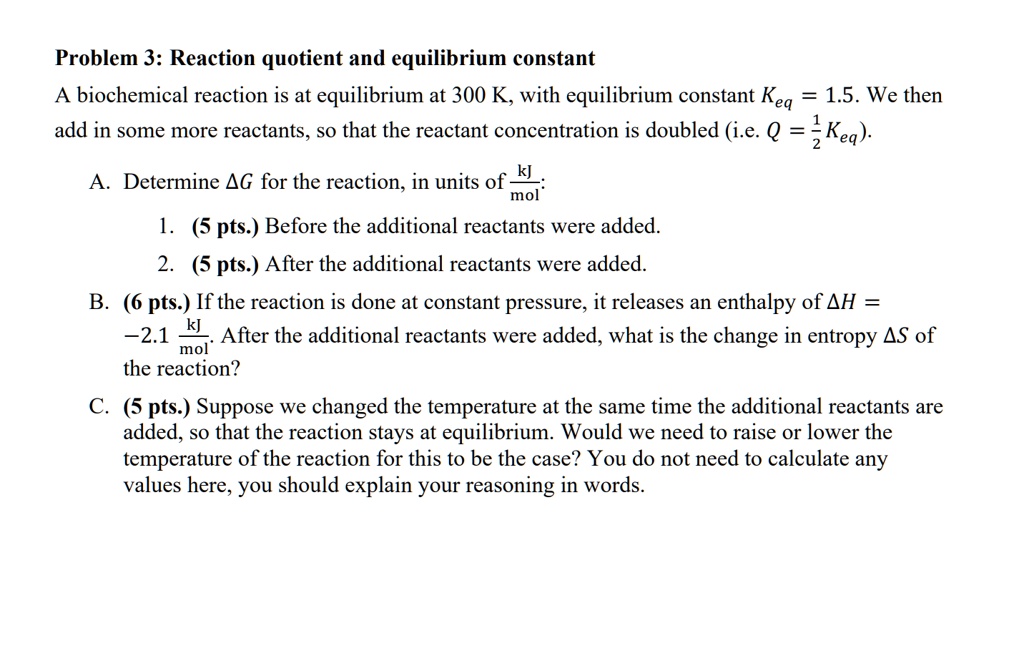
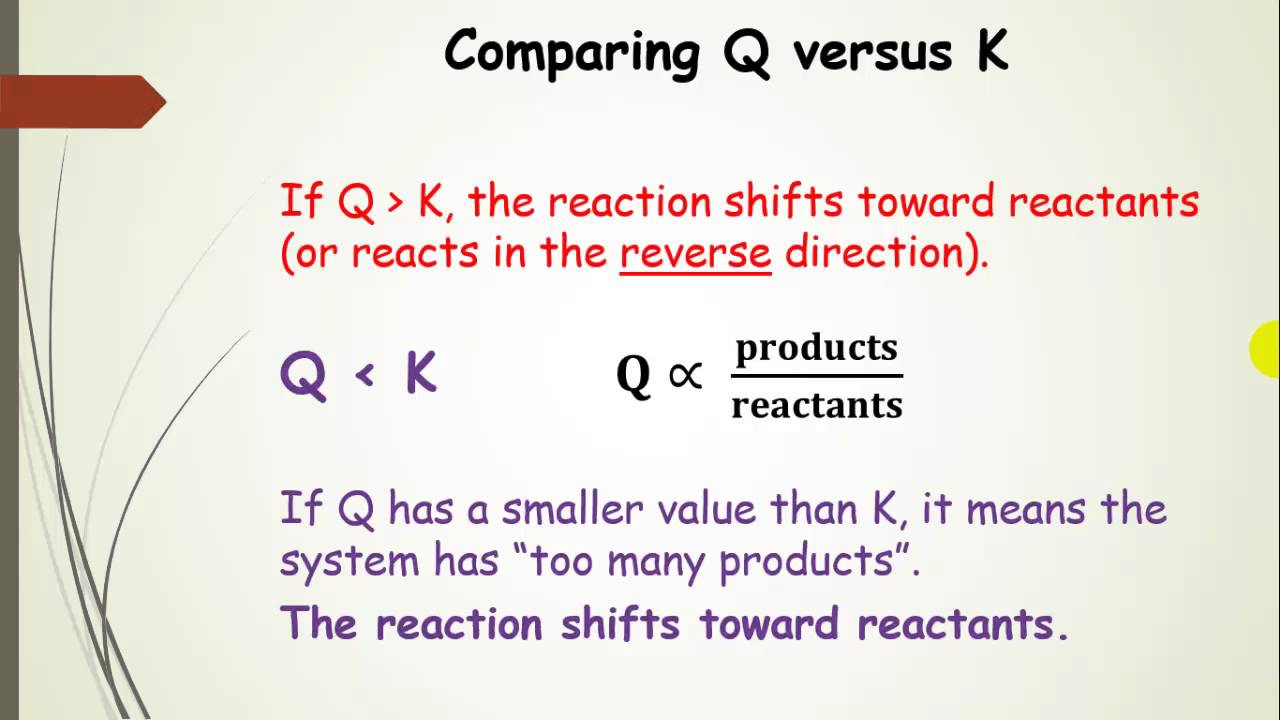
SOLVED: Problem 3: Reaction quotient and equilibrium constant biochemical reaction is at equilibrium at 300 K, with equilibrium constant Keq 1.5. We then add in some more reactants, So that the reactant
![The reaction quotient Q for: N2(g) + 3H2(g) 2NH3(g) is given by Q = [NH3 ]^2 [N2 ] [H2 ]^3 . The reaction will proceed in backward direction, when: The reaction quotient Q for: N2(g) + 3H2(g) 2NH3(g) is given by Q = [NH3 ]^2 [N2 ] [H2 ]^3 . The reaction will proceed in backward direction, when:](https://dwes9vv9u0550.cloudfront.net/images/1971314/5602d142-94f9-4815-ac6e-8c964a4470c6.jpg)
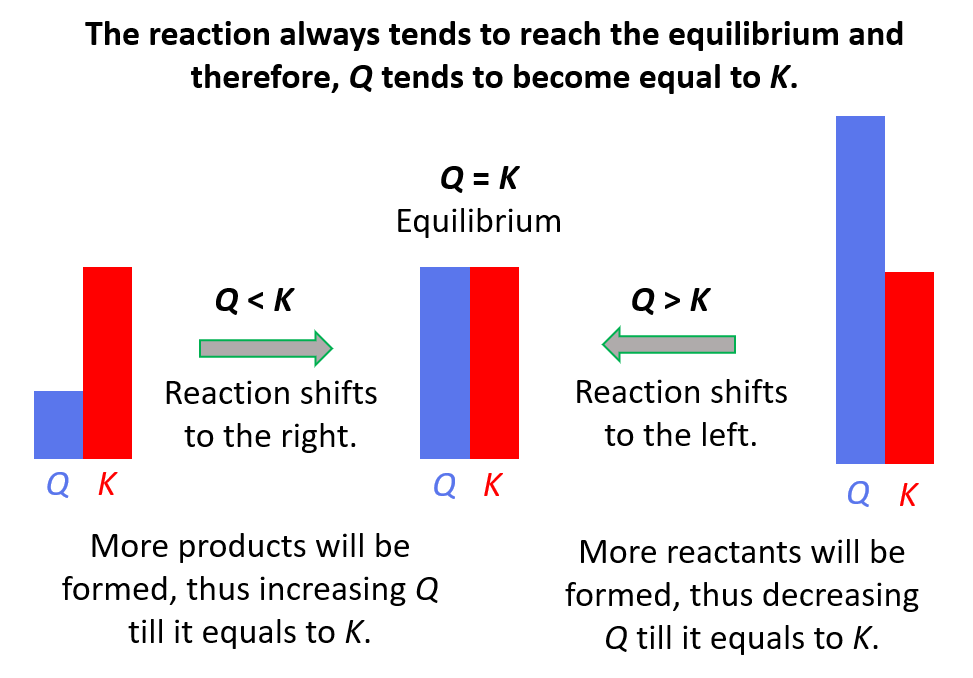
The reaction quotient Q for: N2(g) + 3H2(g) 2NH3(g) is given by Q = [NH3 ]^2 [N2 ] [H2 ]^3 . The reaction will proceed in backward direction, when:
![Quantitative Changes in Equilibrium System. Q, the Reaction Quotient We have written the formula for the equilibrium constant: K = [C] c [D] d [A] a [B] - ppt download Quantitative Changes in Equilibrium System. Q, the Reaction Quotient We have written the formula for the equilibrium constant: K = [C] c [D] d [A] a [B] - ppt download](https://images.slideplayer.com/1/258096/slides/slide_4.jpg)
Quantitative Changes in Equilibrium System. Q, the Reaction Quotient We have written the formula for the equilibrium constant: K = [C] c [D] d [A] a [B] - ppt download
![Quantitative Changes in Equilibrium System. Q, the Reaction Quotient We have written the formula for the equilibrium constant: K = [C] c [D] d [A] a [B] - ppt download Quantitative Changes in Equilibrium System. Q, the Reaction Quotient We have written the formula for the equilibrium constant: K = [C] c [D] d [A] a [B] - ppt download](https://images.slideplayer.com/1/258096/slides/slide_3.jpg)